
“Liquid metal oxidizes when exposed to air or aquatic environments, deterring electrical current. A new “living metal” composite (seen here in a nanoscale view) developed at Binghamton includes bacterial endospores”
ELECTROGENIC BACTERIA
https://nature.com/articles/s41579-019-0173-x
https://advanced.onlinelibrary.wiley.com/doi/10.1002/adfm.202521818
https://techxplore.com/news/2025-11-metal-bridge-biological-electronic
‘Living metal’ could bridge biological and electronic systems
by Chris Kocher / November 5, 2025
“Electronics have been transforming from rigid, lifeless systems into adaptive, living platforms capable of seamlessly interacting with biological environments. Researchers at Binghamton University are pioneering “living metal” composites embedded with bacterial endospores, paving the way for dynamic communication and integration between electronic and biological systems.
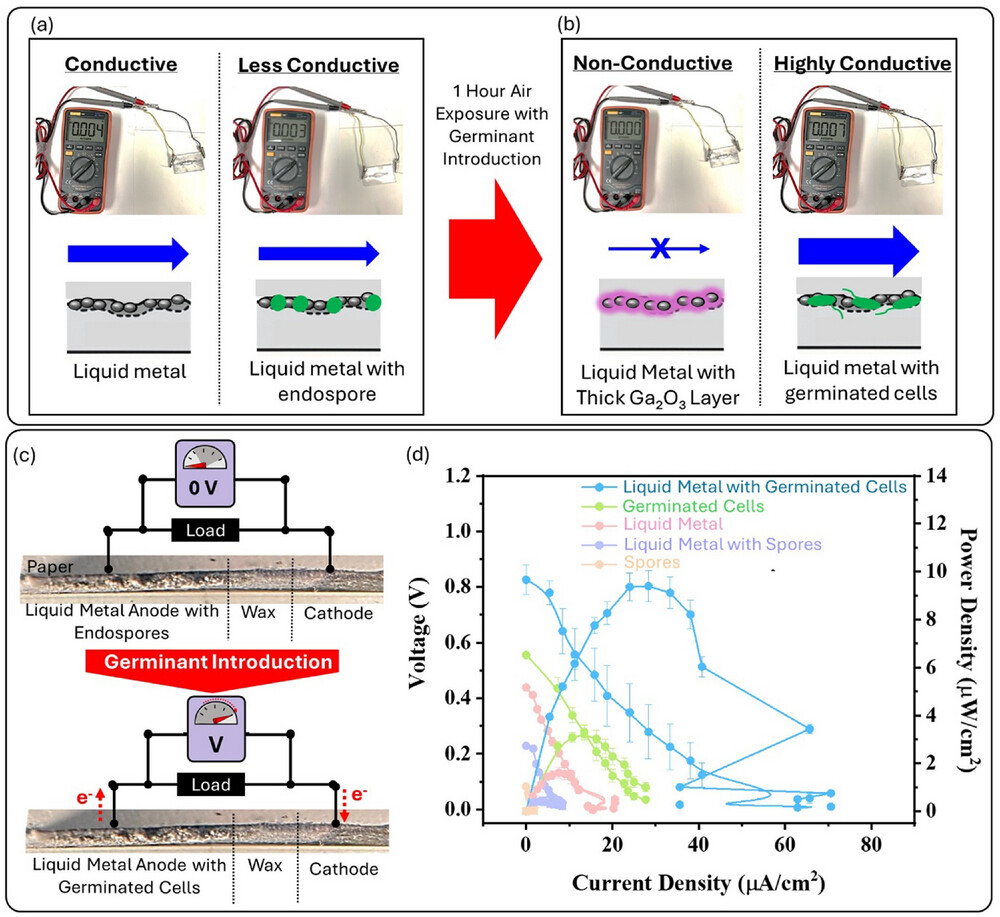
“Conductivity and functionality of the living liquid metal composite”
In a paper published in the journal Advanced Functional Materials, Professor Seokheun “Sean” Choi, Maryam Rezaie, Ph.D., and doctoral student Yang “Lexi” Gao share their potentially groundbreaking study on liquid living metal composites that could redefine the future of bioelectronics. Choi—a faculty member in the Thomas J. Watson College of Engineering and Applied Science’s Department of Electrical and Computer Engineering—is developing innovative technologies to bridge the gap between electronic and biological systems.
Most of Choi’s previous bioelectronic projects employed conductive polymer materials, as liquid metals pose challenges for integration. Their hydrophobic properties hinder adhesion to electronic substrates, and exposure to air or water leads to the formation of an oxide layer that restricts electron flow and disrupts communication between electronic and biological systems. However, he said, polymers have their own difficulties, explaining, “I was not satisfied with the interface—it was not seamless—and although the polymers are conductive, it’s not as much as metal. Also, most bioelectronics will be deployed in very harsh environments, so they are subject to mechanical damage. They must have a self-healing property.”
He believes that electrogenic bacteria—cells which generate small amounts of power—are the key. By combining liquid metal with dormant endospores of the bacteria Bacillus subtilis, which Choi has used to develop biobatteries, the composite material overcomes many of the limitations of liquid metal alone. “When we combine the spores with the liquid metal droplets, there is a huge attractive force, because the spores have chemical functional groups on their surface that interact with the liquid metal oxide layers. This strong force ruptures the oxide layers so the metal can be conductive.”
The spores can stay inactive under harsh conditions and germinate when the environment is more favorable. The composite is also easily absorbed into device substrates such as paper while keeping the best properties of metal. It even exhibits enhanced electrical conductivity when the spores germinate. Most importantly, though, the composite shows the self-healing abilities that researchers want to see. When a break in the material happens, the composite autonomously fills the gap—an important breakthrough when a circuit is damaged and can’t easily be replaced.
Before any commercial applications, more experimentation is needed to better control the activation of the endospores and to evaluate the liquid living metal composites for long-term stability in a variety of environments. In the future, such materials could enable wearable or implantable devices to interface safely and directly with human tissue. “Biological systems use molecules and ions for metabolism or signaling, while electronics exclusively depend on the electrons, so that will create communication errors,” he said. “Electrogenic bacteria use molecules and ions but also generate electrons. The question is how we can seamlessly integrate this electrogenic bacteria into a living electrode to bridge these two systems.”
PREVIOUSLY
LIVE WIRES
https://spectrevision.net/2019/07/04/live-wires/
ELECTRIC BACTERIA
https://spectrevision.net/2014/07/18/electric-bacteria/
SOIL BATTERIES
https://spectrevision.net/2024/01/22/soil-batteries/