“Three researchers sample sediment from the Gowanus Canal
in Brooklyn, NY, aboard a canoe. Credit: Dave Rife”
GOWANUS CANAL SUPERFUND MICROBIOME
https://github.com/HenaffLab/2023_Gowanus_Publication
https://academic.oup.com/jambio/article/136/4/lxaf076/8113800
https://popsci.com/environment/pollution-eating-microbes-gowanus-canal/
Pollution-eating microbes are thriving in infamous NYC canal
by Lauren Leffer / Apr 15, 2025
“For more than 150 years, industrial pollution, chemical waste, and sewage have flowed into Brooklyn’s Gowanus Canal. The New York City waterway is often described as one of the most contaminated in the United States. It was dredged and developed from a tidal wetland and a freshwater creek into its current form in the mid-1800’s, in order to serve as an urban cargo transportation route. Though the paper mills, petroleum plants, tanneries, and manufacturers that once lined its banks are now gone, their legacy of toxic dumping and discharging remains–not to mention the combined sewer overflow that still spills directly into the canal. “It’s accumulated anywhere from ten to twenty feet of contaminated sediment at the bottom of the canal,” says Elizabeth Hénaff, a computational biologist at New York University.
Yet amid all that toxic muck, life finds a way. Microbes in the Gowanus sediment have evolved methods of coping with and even subsisting off of the contamination, according to new research co-authored by Hénaff. Hundreds of hardy microbe species, equipped with dozens of metabolic pathways for breaking down pollution, live at the bottom of the canal, per the study published April 15 in the Journal of Applied Microbiology. These bacteria, archaea, and viruses are slowly sequestering heavy metals and eating their way through some of the worst compounds lingering in the mud.

“Algal and microbial growth in a laboratory aquarium at NYU, emerging from toxic sediment collected from the Gowanus Canal. Photo Credit: Elizabeth Henaff”
The silver lining of the slo-mo environmental disaster is that engineers and biologists might have the chance to harness these superfund superbugs to help detoxify the Gowanus and polluted sites elsewhere. This process known as bioremediation has been applied to challenges like wastewater treatment and cleaning up oil spills, including the massive 1989 Exxon Valdez incident in Alaska. In this way, the “sludge” becomes a “reservoir of potential solutions,” says Sergios-Orestis Kolokotronis, a study co-author and an evolutionary biologist and epidemiologist at SUNY Downstate Health Sciences University.
To discover the canal’s hidden potential, Hénaff, Kolokotronis, and their colleagues conducted the first detailed microbial and genetic survey of the Gowanus. They paddled out onto the infamous waterway and collected sediment samples from the top layer of sunken gunk using a PVC pipe. They also obtained a deeper sediment core from an environmental contractor working with the Environmental Protection Agency (EPA). Simply getting the samples was no easy feat. “It’s not the same thing as going to the park and picking up soil,” says Kolokotronis. “This is dangerous for everyone involved in the canoes,” he adds. In many cases, the researchers had to trespass to access sites. Then, there’s the water and muck itself, which necessitated full personal protective equipment to minimize contact.
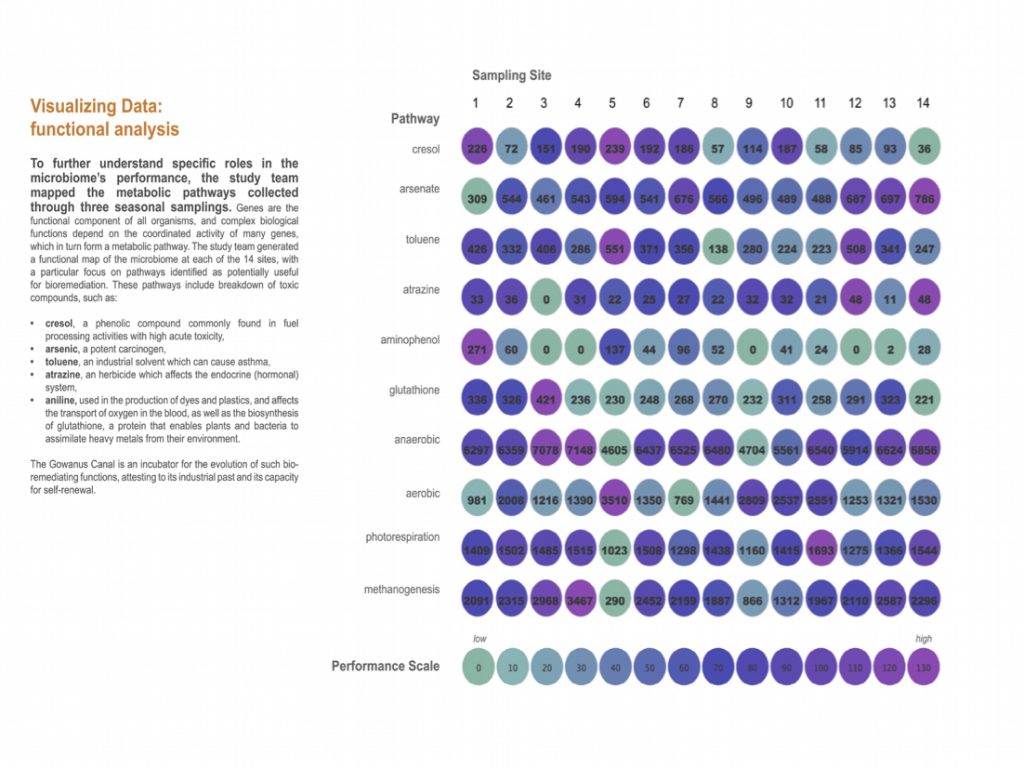
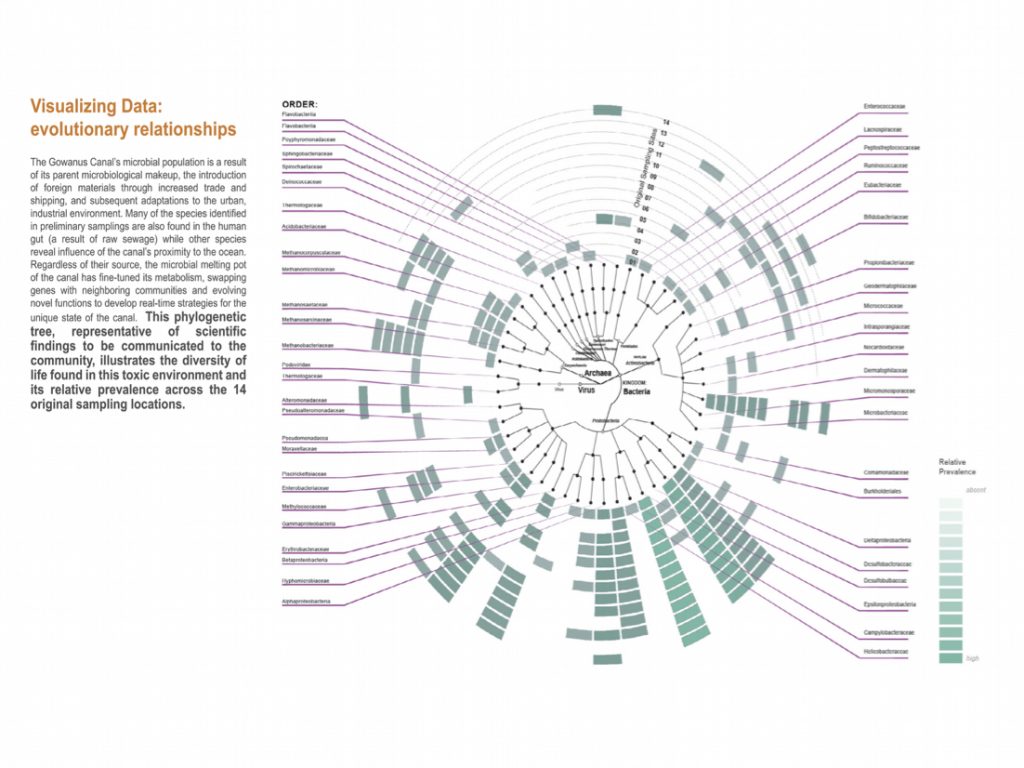
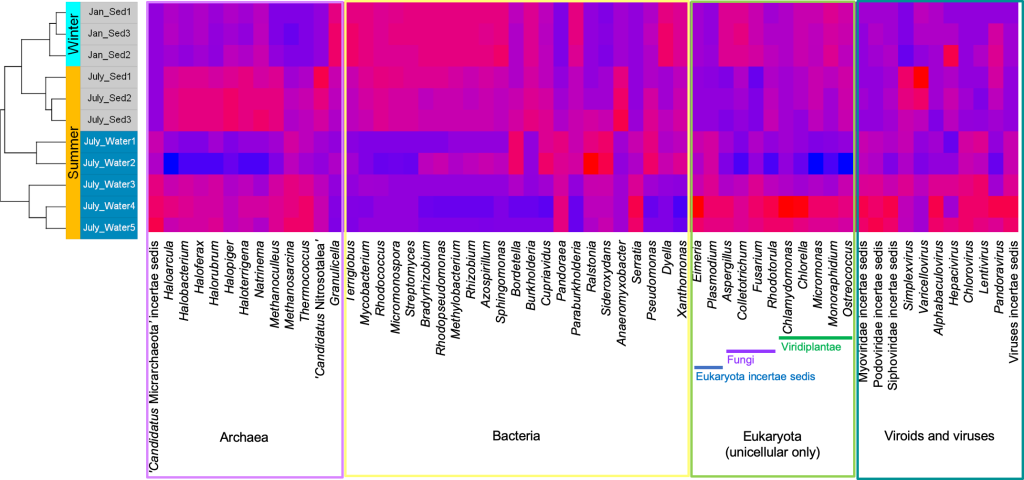
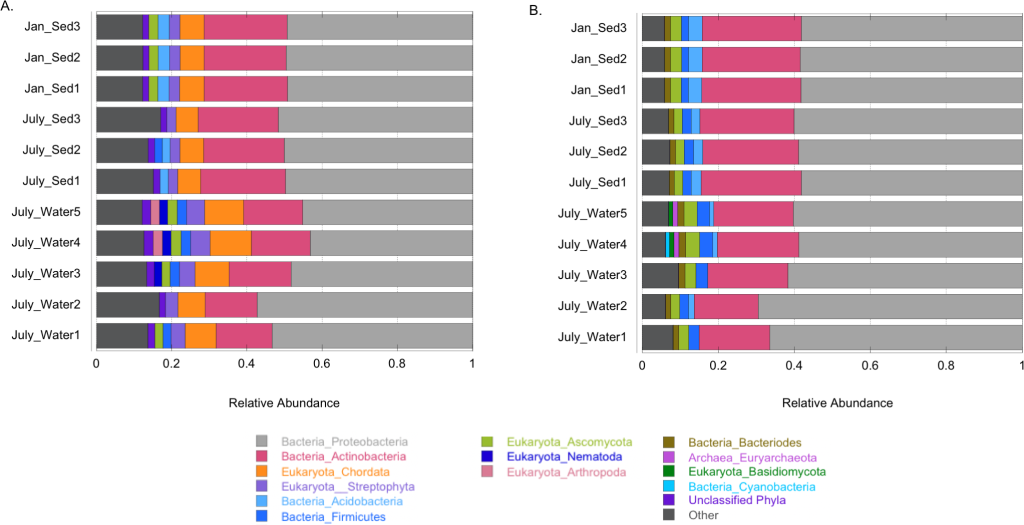
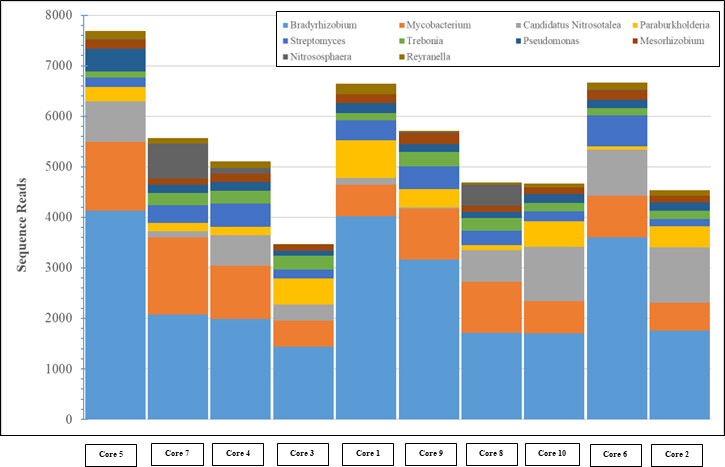
Back in the lab, the scientists analyzed the DNA in these samples to identify the species present and dig into their genetic makeup. They compared the Gowanus data with previously documented organisms and their functions from other databases.The team found 455 different microbe species (including salt and temperature extremophiles), 64 metabolic pathways known to degrade organic contaminants like phenols and toluene, and 1,171 genes related to heavy metal uptake. They also classified thousands of previously un-cataloged gene clusters and metabolites with potential for breaking down pollutants.
It’s the first step in what could be a valuable long-term project, says Max Häggblom, an environmental microbiologist at Rutgers University in New Jersey who was not involved in the new research. The Gowanus is “such a chemical soup,” Häggblom says. “That makes it really interesting for microbiologists because it’s basically a hot spot for selection and evolution of microorganisms with the ability to degrade these different chemicals.” Häggblom agrees the canal could be a useful source of toxin-fighting organisms. But to know for sure, we’d need lab experiments tracking the presence and concentration of pollutants over time in a mini-Gowanus microcosm.

“Sludge growth back in the team’s lab.” Credit: Elizabeth Henaff.”
If the microbes really can break down pollution, they could be “mined” for all sorts of bioremediation projects. Organisms grown and stored in bioreactors might help filter through contaminated water and mud. Alternatively, shifting the environmental conditions of a waterway slightly (for instance, adding the right mix of nutrients) to promote beneficial bacteria could speed up environmental recovery with minimal cost and disruption. Sediment dredged up through the ongoing EPA superfund remediation project might be rendered less dangerous before landfilling by being purposefully mixed and steeped with these helpful bugs.
The findings also act as a ground truth of the types of pollutants in the canal, say Hénaff and Kolokotronis. “These microbially kept records can be more accurate than human kept records,” Hénaff explains. The microbe adaptations prove the presence of toxins that have gone otherwise undocumented in the environmental monitoring and regulation of the waterway. “There’s something poetic about the memory of these organisms,” she adds. In addition to the science, the researchers also synthesized their findings into a public art exhibit about the Gowanus Canal’s history and microenvironment, called CHANNEL.

“Microbial biofilm formed on the surface of contaminated sediment from the Gowanus Canal, grown in an aquarium as part of the art exhibit CHANNEL at BioBAT Art Space in Brooklyn, NY. Photo Credit: Stefan Hagen”
According to Häggblom, it’s marvelous, though not surprising, that these types of microbes routinely emerge under such hostile conditions. Many microorganisms can swap genes with one another, allowing the spread of useful traits without each species having to individually go through the gauntlet of mutation and natural selection. Under the pressure of trying to survive in a toxic environment, and without the standard resources for energy production like oxygen, bacteria and viruses frequently share the alternate solutions they come up with: including methods of using chemicals like PCBs and hydrocarbons to breathe, he explains. “This is nature taking its course.”
Yet despite the wide array of pollution-munching microbes present in the Gowanus, they aren’t capable of fully cleaning up the canal on their own. For one, though many of the microorganisms sequester heavy metals like cobalt and arsenic, the microorganisms can’t actually eliminate those toxic elements. The only way to remove the metals is to scoop them out of the waterway once they’re contained. The microbes could play a role in recycling these metals for new uses. “It’s an exciting possibility,” says Hénaff. Many of the canal pollutants are resources in a different context. “A lot of the heavy metals among the toxic contaminants are being mined elsewhere to serve as a resource for technology and industry,” she notes. Though that, again, requires human-microorganism collaboration.
Other, non-metal, organic pollutants present in the Gowanus can be broken down into non-toxic forms by microbes alone. But not at the ideal speed and scale. Left to their own devices, the microbes would take centuries or even millennia to work their way through the contamination, says Häggblom. As the Gowanus continues to stew in the open, it poses a perpetual public health risk– exposing nearby residents to unhealthy air and soil.
One of the less pleasant study findings was that the canal also harbors a wide variety of genes for antimicrobial resistance. The danger is why the EPA has been working to dredge and cap the Gowanus’ toxic sediments. Ultimately, that’s probably the correct call, says Hénaff. But until the sludge is permanently buried and the microbes dead beneath a concrete tombstone, why not treat the canal as more than a mistake? Why not learn everything we can from the polluted world we accidentally built?”
CITIZEN HAZMAT SCIENCE
https://idm.engineering.nyu.edu/henafflab/gowanus-canal-microbiome-2/
https://brooklynpaper.com/microbes-cleaning-gowanus-canal
https://the-microbiologist.com/microbes-in-brooklyn-superfund-site
Microbes in Superfund site teach lessons on fighting industrial pollution
by Linda Stewart / 15 April 2025
“Using advanced DNA sequence analysis, a research team led by NYU Tandon School of Engineering’s Assistant Professor Elizabeth Hénaff has discovered that tiny organisms in Brooklyn’s highly contaminated Gowanus Canal have developed a comprehensive collection of pollution-fighting genes. The findings were published in the Journal of Applied Microbiology on April 15, 2025. The team identified 455 species of microorganisms wielding 64 different biochemical pathways to degrade pollutants and 1,171 genes to process heavy metals.
This suggests the potential of a cheaper, more sustainable, and less disruptive method for cleaning contaminated waterways than the current oft-used dredging operations. The researchers also discovered 2,300 novel genetic sequences that could enable microbes to produce potentially valuable biochemical compounds for medicine, industry, or environmental applications. “We found what amounts to nature’s own toxic cleanup manual, but with a crucial warning,” said Hénaff, who sits in NYU Tandon’s Technology, Culture and Society Department and is a member of Tandon’s Center for Urban Science + Progress. “These microbes have stories to tell that go beyond scientific data.”

“Gowanus sediment on display in CHANNEL, an immersive installation at BioBAT Art Space in Brooklyn, New York that illustrates the researchers’ microbial study of the waterway. Photo credit: Elizabeth Hénaff”
To communicate these stories effectively, Hénaff and colleagues created CHANNEL, an immersive installation at BioBAT Art Space in Brooklyn, New York featuring sculpture, prints, sound, and projections alongside over 300 gallons of native Gowanus sediment and water that has been growing over the last 9 months. The Living Interfaces Lab, Hénaff’s research group, uses methods from sciences and arts to address pressing urban issues. “While more research is needed to understand how to cooperate with these organisms effectively, the discovery of such genetic tools for pollution cleanup may offer valuable lessons for environmental restoration worldwide,” Hénaff said. “I consider artistic research to be a key component in not just illustrating but also informing our scientific research.” The work is on view at the exhibit’s closing event on April 18, 2025.
The team discovered genes for resistance to eight different classes of antibiotics in the canal microbes, with some coming from human gut bacteria that enter the canal during Combined Sewer Overflows – when heavy rainfall causes stormwater and untreated sewage to discharge directly into waterways. Other resistance genes were found in native aquatic species. “The long-term coexistence of microbial communities from sewage and the natural canal environment is expected to enhance the rates of horizontal transfer of a wide array of genetic elements, and as such merits our attention for public health monitoring and surveillance as environmental ‘superbug’ reservoirs,” said Sergios-Orestis Kolokotronis, a study co-author and assistant professor of epidemiology and infectious diseases at SUNY Downstate Health Sciences University.
Despite these concerns, the study also reveals promising potential benefits. While the pollutant-degrading microbes in the canal can break down contaminants, their natural processes are too slow for practical cleanup. Understanding their genetic adaptations could help scientists develop faster methods, either by isolating specific microbes for treatment or enhancing their abilities. Some classes of contaminants such as heavy metals are also valuable materials for industry, and bioremediation methods could be adapted to resource recovery for re-use, not just removal.
To make its discoveries, the team collected samples from 14 locations along the canal’s 1.8- mile length, gathering both surface sediment and deep core samples reaching 11.5 feet below the canal floor. They found microbes capable of breaking down many historical pollutants, including petroleum products, PCBs, and industrial solvents. The findings come as the Environmental Protection Agency continues its $1.5 billion dredging and capping operation at the canal, removing contaminated sediment and sealing remaining pollution under clean material.
The team’s current study builds on prior research spanning a decade to understand the Gowanus Canal microbiome. The project began in 2014 when the current study’s co-authors Ian Quate of Fruit Studio and Matthew Seibert of the University of Virginia led the first sediment sampling, processing samples at community bio lab Genspace with study co-author Ellen Jorgensen of Biotech without Borders.
The DNA was sequenced in the lab of study co-author Christopher Mason – WorldQuant Professor of Genomics and Computational Biomedicine at Weill Cornell Medicine – as part of the Pathomap Project, now expanded to cities around the world in the metagenomics of subways and urban biomes (MetaSUB) project. “The hardy microbial organisms of the Gowanus Canal have a unique genetic catalog of survival, which provides a roadmap for adaptation and directed evolution that we can use in polluted sites around the world,” said Mason, who serves as co-founder and Director of the MetaSUB Consortium.
Later, lead author Hénaff’s team collected more samples through the BKBioReactor project while study co-author Kolokotronis gathered core samples. Bioinformatic approaches implemented by study co-authors Chandrima Bhattacharya of Weill Cornell Medicine and Rupobrata Panja of Rutgers University allowed the team to identify microbes breaking down industrial pollutants in the canal’s thick sediment.”
SUPERFUND MICROBIOMES
https://epa.gov/superfund/superfund-glossary
https://engineering.nyu.edu/microbes-fighting-industrial-pollution
https://cen.acs.org/Learning-life-living-Superfund-sites/103/web/2025/07
Learning from the life living in Superfund sites
by Bec Roldan / July 14, 2025
“On a cool December day in Brooklyn, New York, in 2014, a group of academic and citizen scientists set off onto one of the most polluted 3 km stretches of water in the US. They’d soon find a thriving community of microbes living in toxic sludge about a meter below the water. One of those keen researchers was Elizabeth Hénaff, who had just joined Chris Mason’s laboratory at Weill Cornell Medicine as a postdoctoral fellow. Several months earlier, two members of the Brooklyn community biology lab Genspace had approached Mason with an intriguing proposal.

“Microbial and algal growth emerging from contaminated sediment of the Gowanus Canal cultured in large-scale aquariums. Photo credit: Elizabeth Hénaff”
They wanted to study the bottom of the Gowanus Canal, an infamously toxic body of water, before the US Environmental Protection Agency dredged its bottom and covered it with an impermeable layer. When she heard about the plan, Hénaff was hooked. Decked out in hazmat suits and rubber boots, Hénaff and the team paddled out into the canal, three people to a boat. When they reached their first sampling location, the person in the back operated a 4 m long polyvinyl chloride pipe, “our super scientific sampling device,” from a big-box hardware store, Hénaff says.
“A group of researchers from Weill Cornell Medicine and Genspace canoe on the Gowanus Canal, with boats and arm power provided by the Gowanus Dredgers Canoe Club, on Dec. 12, 2014, to collect samples of the black sludge at the bottom of the canal. Credit: Elizabeth Hénaff”
After plunging the pipe into the soft sediment of muck below the water and capping the top with a gloved hand, the person at the stern gently pulled it out while maintaining the vacuum. The person at the front of the boat, ready for collection with a 50 mL centrifuge tube in hand, guided the black slop from pipe to tube once the vacuum was released. Back in the lab, the research team extracted and sequenced DNA found in that sludge. “At first it came as a surprise to me that there was anything living in there,” says Hénaff, now an assistant professor in computational biology at New York University. “Now I know. Of course there are microbes there; there are microbes everywhere.”
Since 2014, Hénaff has studied the unseen microbiology of the Gowanus Canal. She and her team recently identified a community of more than 400 different species of bacteria, archaea, and viruses living in that sludge, and more than 1,000 genes that encode for proteins that process heavy metals (J. Appl. Microbiol. 2025, DOI: 10.1093/jambio/lxaf076).). The Gowanus Canal is a particularly contaminated area that the EPA declared a Superfund cleanup site in 2010. The origins of that designation are at Love Canal in Niagara Falls, New York, where during the 1970s, residents experienced high rates of birth defects, pregnancy losses, and cancer as a result of industrial dumping of contaminants like chlorinated hydrocarbons in a local landfill.
The disaster brought the impacts of dumping hazardous waste to the forefront of the environmental movement and garnered widespread attention. To address the environmental and health concerns of hazardous waste sites such as Love Canal, the US Congress passed the Comprehensive Environmental Response, Compensation, and Liability Act in 1980. Part of that act established a $1.6 billion trust to clean up old waste sites, informally called Superfund sites. Abandoned industrial sites contaminated with petroleum chemicals, nuclear waste, pesticides, heavy metals, and other anthropogenic pollutants are poisoning the environment and wreaking havoc on public health.
But while most organisms die off in such extreme environments, some microorganisms thrive. “These extremophiles have specific adaptations that let them tolerate the particular conditions they are in,” says Jeffrey Morris, an associate professor in microbiology at the University of Alabama at Birmingham. And in the case of polluted environments, those adaptations allow microbes to tolerate and degrade or otherwise detoxify environmental contaminants. “Bacteria grow really fast and can adapt to almost any kind of environment you throw at them, anything that doesn’t just kill them out right,” Morris says. “If you give them time around a pollutant, they’ll come up with solutions to grow better in its presence.”
As researchers focused on hazardous waste sites, they began leveraging these microbes for cleanup, a process known as bioremediation. Early uses relied on microbes to clean up oil spills, such as the 1989 Exxon Valdez spill and the 2010 BP Deepwater Horizon spill. Because oil exists naturally in the environment, microbial communities that know how to consume components of oil already exist: Oceanospirillales bacteria, which use hydrocarbons as a source of carbon and energy, are one example. Microbes in sites full of human-made pollution have more pressure to evolve.
Each Superfund site is unique, with a distinct pollution history and dominant contaminants that offer scientists a “window into the process of evolution itself,” Morris says. What’s more, Morris and other researchers say, as they find new bacteria that survive in polluted sites, they can perhaps put those microbes to work. One day they may clean up pollution and do important, more sustainable chemistry. At some sites, bioremediation efforts are already under way. In New York, Hénaff’s team identified 455 freshwater and saltwater species of bacteria, archaea, and viruses, and identified 64 ways microbes degrade organic pollutants and 1,171 genes that encode for proteins that use or detoxify heavy metals.
“The K-25 Gaseous Diffusion Plant campus, Oak Ridge, Tennessee, in 2019 during the demolition process. The site is contaminated with the common industrial solvent trichloroethylene.”
Hénaff identified microbes that can live in extremely salty environments, like sulfate-reducing Desulfobacterium autotrophicum, and heavy metal–contaminated environments, like Microbacterium laevaniformans. Her team also observed bacteria typically found in the human gut, which aligns with the frequent sewage overflows into the canal. Hénaff says you can see, via the microbes that the team detected, how the Gowanus Canal was plagued by industrial waste dumping and commercial shipping activities, resulting in a chemical soup of infamous pollutants. “One interesting takeaway is this idea of microbial memory that’s maintained by these nonhuman organisms,” Hénaff says. “It’s a memory of the history of human intervention in a site.”
As a kid growing up in Brooklyn, just a few miles away from the Gowanus Canal, Lesley-Ann Giddings knew to avoid the notoriously toxic water. Years later, as a biochemistry professor at Middlebury College, Giddings set out to explore a different Superfund site, one plagued not by urban industrial pollution but by the legacy of mining that has left a microbial mark. The Ely Copper Mine located in the old Copper Belt region of Vermont is home to abandoned mining-waste piles that are packed with rocks rich in metal sulfides. The rock piles drain acidic water into surrounding groundwater and sediment, a process known as acid rock drainage. Water in this region is contaminated with toxic levels of copper, iron, magnesium, zinc, and lead, and in 2001, the EPA designated it a Superfund site. Intrigued by the possible microbial communities thriving in the hyperacidic environment, Giddings decided to go microbe hunting
“Acid rock drainage carries sulfides in the Ely Brook to the Schoolhouse Brook on May 7, 2025, in Vershire, Vermont. Lesley-Ann Giddings hopes to find bioactive compounds by studying the microbial community in the hyperacidic environment of the Ely Brook at the Ely Copper Mine site. Credit: US EPA”
The bright orange soil that clung to her boots as she stepped out of her car and made her way past the mine tailings, a by-product of mining, made it “very clear that we were at this mine with a lot of oxidized metals,” says Giddings, now a professor at Smith College. Giddings focused on a brook near the mine’s entrance and, like Hénaff, relied on a DIY approach to collect samples for DNA sequencing.
On a sunny summer day in 2015, Giddings and a small team hooked up a peristaltic pump to an old car battery, allowing them to pump water from the brook through filters that captured DNA. They later returned to the site a few more times over the next 4 years, in the winter and summer. “The acid rock drainage environment is very nutrient deficient,” Giddings says, so to identify the acid-loving microbes surviving in this inhospitable environment, her team used shotgun metagenomic sequencing, an analysis that sequences all microbial genomes in a sample.
Hénaff’s team relied on the same sort of genetic analysis to make sense of the Gowanus sludge. To prepare samples for sequencing, the cellular membranes of the cells are cut open to release their DNA, which is then separated from cellular debris and chopped into pieces short enough for a sequencing instrument to handle. Hénaff describes the process as taking a “mixed bag of bacteria and their genes,” from a site sample and processing it down to “a mixed bag of small pieces of DNA, each 150 base pairs long.” After that, a DNA-sequencing instrument turns molecules into data ready for computational analysis, comparing data from the mixed bag of DNA fragments with those in databases listing the unique genetic material specific to a certain microbe or assigning function to specific genes.
The Ely Brook microbiome that Giddings pieced together revealed a community of acid-tolerant bacteria, including Proteobacteria and Actinobacteria commonly found in metal-rich environments (PLOS One 2020, DOI: 10.1371/journal.pone.0237599). The team also identified bacteria that oxidize iron and sulfur, which are in high concentrations in the brook, as well as others, like Bradyrhizobium species, which produce nutrients for plants by reducing nitrogen gas to usable ammonia. Other researchers have found Bradyrhizobium bacteria at a former nuclear weapons production facility, the Savannah River Superfund site.
But Giddings notes that the environment could have more microbes and genes that she wasn’t able to identify. Metagenomic analyses rely on previous research catalogued in existing databases to identify microbes and assign function to genes in a given sample, and because acid rock drainage environments are understudied, Giddings thinks there may be genes or microbes the analysis wasn’t able to label. Beyond identifying these pollution-gobbling microbes, understanding what they actually do in the presence of pollutants could pave the way for their use in bioremediation. In the 1990s, scientists discovered that waste- and groundwater contaminated with a common industrial solvent, trichloroethylene (TCE), contained Dehalococcoides bacteria. These bacteria dechlorinate TCE, now a known human carcinogen that the EPA recently banned, and convert it to nontoxic ethene.
Dehalococcoides species “use chlorinated solvents as their electron acceptor, the same way that you and I use oxygen,” says David Freedman, an environmental engineering professor at Clemson University. “We now understand there are dechlorinating bacteria that breathe hundreds of different types of chlorinated organics,” he says, including chlorinated methanes and polychlorinated biphenyls. Dehalococcoides cultures are now commercially available for bioremediation projects that need to break down toxic chlorinated ethenes.
Unlike organic pollutants, heavy metals can’t be fully degraded, but they can be isolated and even transformed into less toxic versions. Certain microbes get rid of toxic metals by using proteins to pump out unwanted materials, “so if the metal ends up inside the cellular membrane of the microbes, they have the capacity to pump it back out” Hénaff says. Other microbes immobilize the metals by absorbing them into bacterial cell surfaces or binding them inside cell walls with proteins. Microbes that hyperaccumulate heavy metals could one day be used to capture precious metals like lithium from the environment for reuse. “What’s considered a contaminant in this environment is a resource in other environments,” Hénaff says.
Microbes can also manipulate the oxidation state of metals to convert them into insoluble, immobile, and nontoxic states. “Oxidation states mean everything with respect to the mobility and toxicity of heavy metals,” Freedman says. For example, iron-reducing microbes like Geobacter metallireducens can convert hexavalent chromium, a carcinogenic industrial compound shown to cause lung cancer, into insoluble, nontoxic trivalent chromium. Other microbial species dump waste electrons onto pentavalent arsenic, reducing it to soluble and more toxic trivalent arsenic. Giddings identified certain microbial genes in the Ely Brook that reduce sulfates into sulfides.
Several strategies exist for cleaning up contaminated Superfund sites, but Freedman says bioremediation is a favored approach for several reasons. One in particular stands out: “If you can accomplish remediation using biology, it’s going to be cheaper than using physical or chemical processes,” he says. Ideally, remediation experts could just monitor how native microbial communities are dealing with pollutants on their own, but microbes can be slow, especially if their environment isn’t set up to maximize pollution degradation. So that’s when they step in to help. Remediation specialists can encourage microbes to move faster by pumping in nutrients to create the ideal conditions for cleanup. At the East Tennessee Technology Park, Roger Petrie and Sam Scheffler from the US Department of Energy’s Oak Ridge Office of Environmental Management are focused on doing just that.”
PREVIOUSLY
URBAN MICROBIOMES
https://spectrevision.net/2025/08/06/metal-breathing-microbes/
SEAFLOOR DUMPSITES
https://spectrevision.net/2021/05/05/seafloor-dumpsites/
MICROBES as GEOACTIVE AGENTS
https://spectrevision.net/2020/10/01/radical-geomycology/
SUB-LETHAL METABOLIC EFFECTS
https://spectrevision.net/2018/04/02/sub-lethal-impacts/
RECREATIONAL GENETICS
https://spectrevision.net/2015/05/08/recreational-genetics/
UNNATURAL SELECTION
https://spectrevision.net/2013/05/24/unnatural-selection/