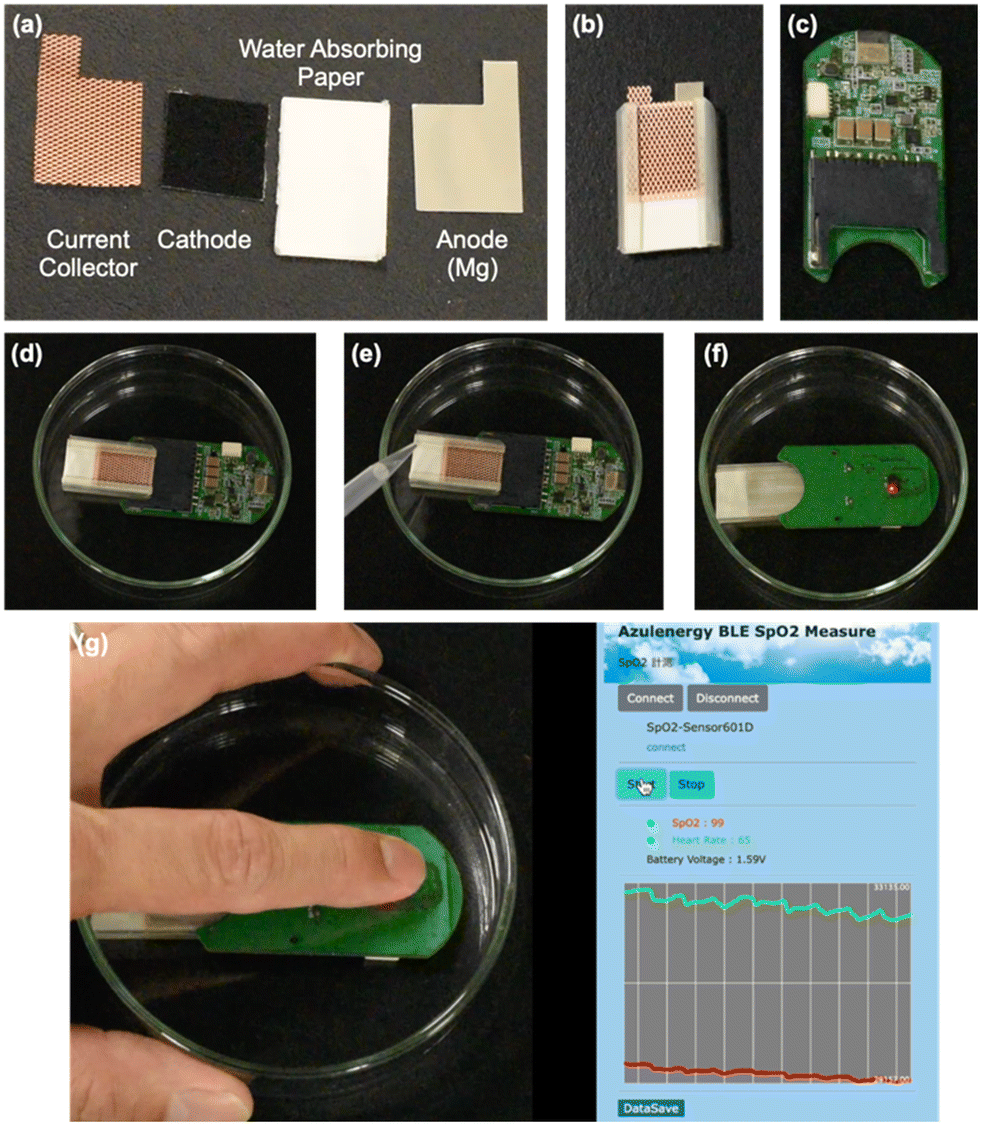
“Photographs of cell components (a), a single paper battery cell (b), SpO2 sensor (c), SpO2 sensor with the cell (d), injection of salt water to the cell (e), and the active SpO2 sensor (other side compared with panels (d) and (e)) (f). Real-time monitoring of O2 saturation and pulse measured in a person (g).”
Mg-AIR BATTERIES
https://pubs.rsc.org/en/content/articlelanding/2024/lf/d4lf00039k
https://techxplore.com/metalair-paper-battery-wearable-devices
Inspiration from plants: Metal–air paper battery for wearable devices
by Tohoku University / April 3, 2024
“For more than two millennia, paper has been a staple of human civilization. But these days, the use of paper is not limited to writing. It is also playing a pivotal role in ushering in a greener future. Lightweight and thin paper-based devices help reduce dependence on metal or plastic materials, while at the same time being easier to dispose of. From paper-based diagnostic devices that deliver economical and rapid detection of infectious diseases to batteries and energy devices that offer an environmentally friendly alternative for power generation, scientists are finding ingenious ways to put this versatile material to use.
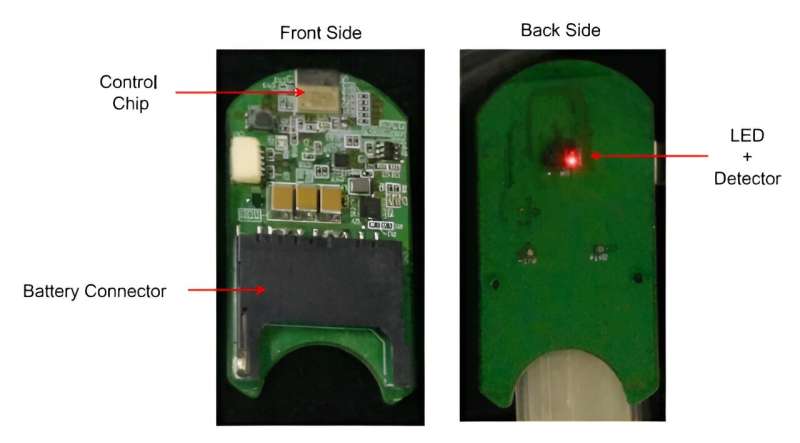
“Photographs and a circuit diagram of a SpO2 sensor without cover. On the front side, control IC chip and battery connector were equipped. On the back side, the LED and detector for measuring pulse and O2 saturation were equipped.”
Now, a team of researchers at Tohoku University has reported on a high-performance magnesium–air (Mg–air) battery that is paper-based and activated by water. Details of their research were published in the journal RSC Applied Interfaces on March 18, 2024. “We drew inspiration for this device from the respiration mechanism of plants,” says Hiroshi Yabu, corresponding author of the study. “Photosynthesis is analogous to the charge and discharge process in batteries. Just as plants harness solar energy to synthesize sugar from water in the ground and carbon dioxide from the air, our battery utilizes magnesium as a substrate to generate power from oxygen and water.”

“SEM images of water-absorbing paper sheets Surface structures of paper sheets were observed by using a scanning electron microscope (SEM, S-5200, Hitach HighTech, Hitachi, Japan).”
To fabricate the battery, Yabu and his colleagues bonded magnesium foil onto paper and added the cathode catalyst and gas diffusion layer directly to the other side of the paper. The paper battery achieved an open circuit voltage of 1.8 volts, a 1.0 volt current density of 100 mA/cm-2, and a maximum output of 103 milliwatts/cm-2. “Not only did the battery demonstrate impressive performance results, it operates without using toxic materials—instead using carbon cathodes and a pigment electrocatalyst that have passed stringent assessments,” adds Yabu. The researchers put the battery to the test in a pulse oximeter sensor and a GPS sensor, illustrating its versatility for wearable devices.”
More information: Kosuke Ishibashi et al, Rare-metal-free high-performance water-activated paper battery: a disposable energy source for wearable sensing devices, RSC Applied Interfaces (2024). DOI: 10.1039/D4LF00039K
METAL-AIR BATTERIES
https://sintef.no/sustainable-energy/metal-air-batteries/
https://interestingengineering.com/paper-magnesium-battery-power
Researchers develop paper battery that generates power from water, air
by Maria Mocerino / Apr 05, 2024
“Step aside lithium and alkaline. Magnesium might be the next power player in the battery world. Studies have shown that a magnesium-based energy storage device could be safer and more powerful than its metal competitors. The Earth has more Magnesium than Lithium. Magnesium ions also have a higher energy density and two positive charges. Lithium ions are smaller and have a single positive charge. Most might be familiar with lithium and lithium-ion batteries. They’re found in flashlights, cameras, security systems, and even cell phones, tablets, vaping devices, tools, e-bikes, and Metal-air batteries use oxygen to generate electricity.
The magnesium-air battery (Mg-air) from Tohoku University is innovative, however. The researchers made it out of paper, first of all, and it runs on water. In a fascinating new study, researchers documented that the respiratory system of plants inspired them to make the Mg-air battery. “We thought that,” the authors of the study write, “if a system in which paper absorbs electrolyte by capillary force, like plants suck up water from the ground, could be applied to magnesium–air batteries, the problem of magnesium dissolving in the electrolyte and self-discharging would be solved. “The battery performance depends on the water absorbent paper,” in other words.
Paper-based devices reduce the use of metals and plastics, so this magnesium-air battery poses the least threat to the environment. Metal-air batteries, typically, regardless, use heavy metals. They can’t just be thrown away, even if they contain heavy metals in small quantities. They’re still dangerous for the environment. Paper-based batteries are disposable. Finally, the paper-based magnesium-air battery has a high performance output. One of the hurdles scientists have faced with using magnesium as an energy source is its low mobility rate. Lithium ions move faster.
Studies have already shown that magnesium ions can be manipulated to have a faster mobility rate. After all, no one wants to wait for hours and hours for their electronics to charge. This paper-based magnesium-air battery thus proves itself on two levels. It could become the first eco-friendly energy storage device that meets high-performance standards. A material as thin as paper might just blow the heavyweight metals away.”
“The water battery prototype.” (Carelle Mulawa-Richards, RMIT)
WATER BATTERIES
https://onlinelibrary.wiley.com/doi/10.1002/adma.202400237
https://sciencealert.com/water-batteries-cheaper and-wont-explode
New ‘Water Batteries’ Are Cheaper, Recyclable, And Won’t Explode
by Clare Watson / 05 March 2024
“Water and electronics don’t usually mix, but as it turns out, batteries could benefit from some H2O. By replacing the hazardous chemical electrolytes used in commercial batteries with water, scientists have developed a recyclable ‘water battery’ – and solved key issues with the emerging technology, which could be a safer and greener alternative. ‘Water batteries’ are formally known as aqueous metal-ion batteries. These devices use metals such as magnesium or zinc, which are cheaper to assemble and less toxic than the materials currently used in other kinds of batteries.
Batteries store energy by creating a flow of electrons that move from the positive end of the battery (the cathode) to the negative end (the anode). They expend energy when electrons flow the opposite way. The fluid in the battery is there to shuttle electrons back and forth between both ends. In a water battery, the electrolytic fluid is water with a few added salts, instead of something like sulfuric acid or lithium salt. Crucially, the team behind this latest advancement came up with a way to prevent these water batteries from short-circuiting. This happens when tiny spiky metallic growths called dendrites form on the metal anode inside a battery, busting through battery compartments.
Although the new technology is unlikely to replace lithium-ion batteries any time soon, with further research and development, water batteries could provide a safe alternative to lithium-ion ones in a decade or so, says lead author, chemical scientist Tianyi Ma of RMIT University in Melbourne, Australia. Lithium-ion batteries, which are found in everything from laptops and phones to electric bikes and cars, can overheat and catch on fire in extreme cases. This is because lithium is quite an active metal, which is submerged in an organic electrolyte. Owing to these safety concerns, researchers have long been trying to devise batteries that use different materials but produce the same performance and have similar longevity.
A major hurdle to using aqueous metal-ion batteries has been dendrite growth. To inhibit this, the researchers coated the zinc anode of the battery with bismuth metal, which oxidizes to form rust. This creates a protective layer that stops dendrites from forming. The feature also helps the prototype water batteries last longer, retaining more than 85 percent of their capacity after 500 cycles, the researchers’ experiments showed. According to Royce Kurmelovs at The Guardian, the team has so far developed water-based prototypes of coin-sized batteries used in clocks, as well as cylindrical batteries similar to AA or AAA batteries. The team is working to improve the energy density of their water batteries, to make them comparable to the compact lithium-ion batteries found inside pocket-sized devices.
Magnesium is their preferred material, lighter than zinc with a greater potential energy density. Ma says if magnesium-ion batteries can be commercialized, the technology could replace bulky lead-acid batteries within a few years. Lead-acid batteries have a low energy density, and are used to start petrol or diesel car motors and in large-scale grid energy storage. However, because they contain lead and hazardous acids, they cannot be disposed of and must be recycled at specialist facilities. Recycling or reusing lithium-ion batteries is also a top priority given projected increases in demand for the batteries and the metals used to make them, as the world electrifies its energy systems to phase out fossil fuels and combat climate change.
“Addressing end-of-life disposal challenges that consumers, industry, and governments globally face with current energy storage technology, our batteries can be safely disassembled and the materials can be reused or recycled,” Ma says. In terms of practical applications, the researchers hooked their battery design up to a solar panel and a 45-watt solar light, which the battery kept illuminated for 12 hours after a day’s charge. It’s a small-scale demonstration of the potential of ‘water batteries’ to be used for renewable energy storage, which should encourage more research. The study has been published in Advanced Materials.”
PREVIOUSLY
IRON-AIR BATTERIES
https://spectrevision.net/2012/08/03/low-cost-energy-storage/
NATURAL BATTERIES
https://spectrevision.net/2023/05/03/hacking-photosynthesis/
SOIL BATTERIES
https://spectrevision.net/2024/01/22/soil-batteries/